History
Country Background
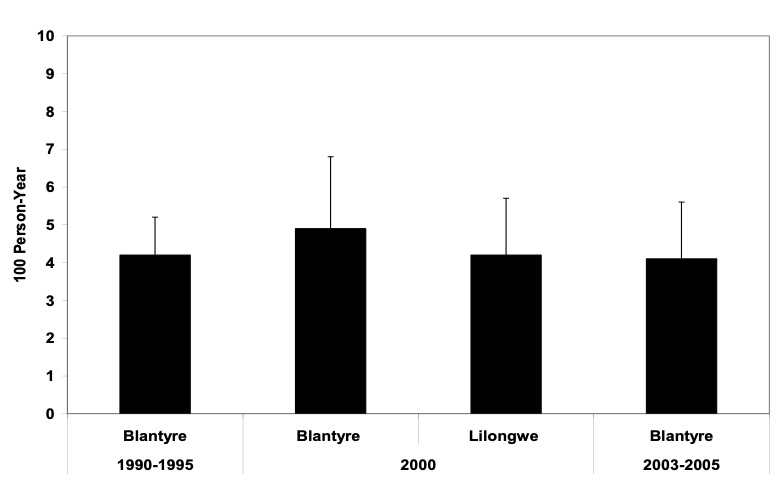
Malawi is a country with a population of about 12 million in Southern Africa, bordered by Mozambique, Tanzania and Zambia. The country is landlocked and about 20% of the surface area is covered by Lake Malawi. The capital is Lilongwe in the Central region and the biggest commercial center is Blantyre in the Southern region. About 80% of the population lives in rural areas engaged in subsistence farming. The per capita income in Malawi is approximately USD 160 with more than half of the population living below the poverty line (<USD 1.0 a day). According to the Demographic and Health Survey in 2000 (1), the infant and child mortality rates were 103.8 and 188.6 per 1000 live births, respectively. AIDS was first detected in Malawi in 1985. By end of 2003 it was estimated that the national HIV prevalence in Malawi was 14% in adults 15-49 years old, and 900,000 adults and children were living with HIV. The demographic impact of HIV/AIDS in Malawi has been substantial with approximately 200,000 dying from AIDS (and co-morbidities) and the life expectancy dropping from 56 to 38 years. The most important mode of HIV transmission in Malawi is heterosexual, accounting for approximately 90% of infections. The predominant virus is HIV-1, more than 95% subtype C (2). Figures 1-2 show HIV-1 prevalence and incidence among women of reproductive age in Malawi. These data suggest that HIV prevalence has leveled off, and probably showing trend of decline. HIV incidence, however, has remained stable and not declined.
Figure 1: HIV-1 Prevalence among antenatal & intrapartum women, Blantyre, Malawi
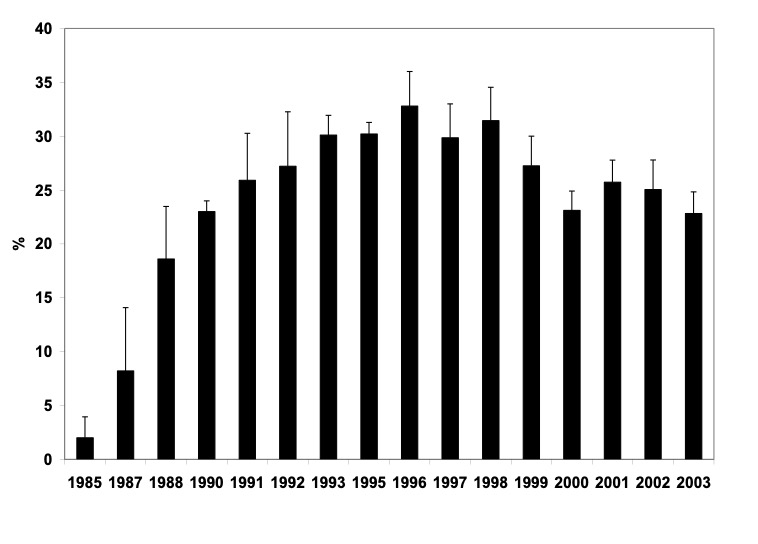
Figure 2: HIV-1 Incidence among antenatal & postnatal women, Blantyre & Lilongwe, Malawi
Research conducted by our Malawi HRC.
The Johns Hopkins University (JHU) - Ministry of Health (MOH)-College of Medicine (COM)
Research Project in Blantyre, Malawi
JHU has been conducting HIV/STD research in Malawi since 1988. The Project started in collaboration with the Malawi MOH and later, the Malawi COM joined this collaboration (the COM was established in 1991). This long-standing interaction between JHU, the MOH and the COM has helped to establish a strong and stable relationship. This collaborative research project has carried-out several studies on HIV and STDs in a systematic manner. The earlier studies were descriptive and observational, involving the investigations of prevalence and incidence of HIV/STDs, risk factors for HIV infection, rates of mother to child transmission of HIV, and the impact of HIV/AIDS on mothers and children. Subsequently, clinical trials, based on the information that has been accumulated, were conducted in the fields of heterosexual and perinatal HIV prevention. More recently, collaborative research to conduct HIV vaccine trials in Malawi has been established. This logical sequence of activities has been well received by policy makers in Malawi as well as by the international scientific community.
A major accomplishment of the JHU-MOH-COM Research Project in Malawi is the establishment of a research site capable of conducting multiple large studies including clinical trials of HIV preventions and vaccines. An experienced core team of researchers (e.g., clinicians, nurses, counselors, data managers) and a laboratory capable of conducting all conventional and non-conventional HIV/STD tests are now available in Malawi.
Research
The studies conducted (completed or in progress) by the JHU Research Project in Malawi are summarized in Tables 1 and 2:
Table 1: Observational HIV Studies Conducted by the JHU-COM-MOH Research Project
| Year | # Screened (Enroll.) | Objectives of the Study |
| 1989-90 | 6,603 (1,366 mothers) | Determine HIV prevalence, incidence and risk factors & MTCT (ICAR). |
| 1993 | 2,471 (1,100 mothers) | Study HIV seroconversion rates (PAVE). |
| 1994-95 | 1,690 (1,690 men) | Determine HIV incidence among men working in sugar estate. |
| 1995-97 | 2,013 (1,633 mothers) | Study risk factors for HIV/STDs (HIVNET). |
| 1995-97 | 853 (853 infants) | Study HIV transmission due to breastfeeding (HIVNET). |
| 1996 | 1,200 (1,020 mothers) | Determine the effect of HIV infection on maternal morbidity/mortality. |
| 1998 | 1,600 (1,600 men) | Monitor HIV incidence among men working in a sugar estate. |
| 1998-01 | 20 (20 men) | Study virological and immunological factors that could influence HIV vaccine use (HIVNET 028). |
| 1999-02 | 1400 (830 mothers) | Study epidemiology and microbiology of mastitis and MTCT. |
| 2004 | 2,142 (2,142 adults) | Prevalence/Incidence of HIV/STDs in a sugar estate. |
Table 2: Intervention HIV Studies Conducted by the JHU-COM Research Project
| Year | # Screened (Enroll.) | Objectives of the Study |
| 1994-95 | 6,964 (6,964 pregnant women/Infants) | Effect of washing birth canal with chlorhexidine to prevent MTCT of HIV and other infections. |
| 1995-98 | 3,949 (985 women/Infants) | Effect of prenatal vitamin A supplement on MTCT of HIV (MCH Study). |
| 1997-98 | 159 (20 women) | Phase I safety study of the microbicide BufferGel. |
| 1998-02 | 139 (60 women) | Determine the efficacy of N-9 gel in reducing HIV incidence. |
| 1998-03 | 1,000 (1,000 women) | Intensive condom counseling and promotion to increase uptake & reduce STIs (HPTN 016/016A). |
| 1998-04 | 3,100 (570 pregnant women) | Determine efficacy of antibiotic treatment of chorioamnionitis in reducing MTCT of HIV (HPTN 024). |
| 1999-04 | 2,650 (2,100 pregnant women/Infants) | Determine efficacy of short antiviral regimen (AZT/NVP) on MTCT of HIV (NVAZ Study). |
| 1999-05 | 4,500 (800 adults) | Determine daily supplementation of vitamin A effect in conjunction with TB therapy on outcome of HIV/TB. |
| 2003-05 | 2,600 (1664 women) | Determine efficacy of intermittent antibiotic intravaginal gel treatment in reducing the incidence of BV (METRO Study). |
| 2003-04 | 150 (50 men) | Safety and acceptability of a penile microbicide wipe |
Description of the JHU-COM Clinical Research Site in Blantyre
The current JHU-COM Research Project in Blantyre is located within the Queen Elizabeth Central Hospital (QECH). QECH is the largest hospital in Malawi with about 1000 beds. It is the main teaching hospital for the COM with specialties in all fields of medicine, pediatrics, obstetrics and gynecology and surgery. The hospital has outpatient and referral clinics, including clinics for antiretroviral treatment (ARV) and STIs. The COM faculty provides clinical services in these clinics, in addition to training of medical students and residents. Currently, the JHU-COM Research Project employs about 120 individuals, and with the exception of 4 expatriate research coordinators, all are Malawian nationals working as research coordinators, nurses, data managers, data clerks, technicians, counselors, and support staff. They are stationed at the Queen Elizabeth Central Hospital (QECH) in Blantyre (the main referral and teaching hospital in the country), and in 7 health centers and clinics in and around Blantyre city in southern Malawi. The COM-JHU Blantyre administrative offices are located in a new building on the grounds of the QECH.
College of Medicine Faculty Currently Participating in On-Going Johns Hopkins University Research in Malawi
- Newton Kumwenda MSc, MPH PhD, Epidemiologist (Fogarty Fellow): Field Director of the JHU-COM Research Project in Malawi, and Faculty COM, University of Malawi and JHU Bloomberg School of Public Health. Dr Kumwenda is resident in Malawi and manages all research and administrative activities.
- George Kafulafula MBBS MMed, Obstetrician and Gynecologist (Fogarty Fellow): Senior Lecturer COM, University of Malawi: Investigator of Record, HPTN024 Perinatal Clinical Trial and Co-investigator HPTN035 Vaginal Microbicide Clinical Trial.
- Johnstone Kumwenda MBChB MRCP, Internist (Fogarty Fellow): Senior Lecturer COM, University of Malawi: Investigator of Record, HPTN052 Antiretroviral Clinical Trial and Co-investigator HPTN024 Perinatal Clinical Trial.
- Thomas Ndovi PhD, Internist (Fogarty Fellow): HIV Vaccine Coordinator and Investigator of Record, HVTN050 HIV Vaccine Clinical Trial.
- George Liomba MBBS FRCPath, Pathologist: Professor COM, University of Malawi: In-Country Principal Investigator of the HPTN and Co-investigator HPTN024, HPTN035, HVTN050.
- Chiwoza Bendawe PhD, Behavioral Scientist: Senior Lecturer COM, University of Malawi (Fogarty Fellow): Co-Investigator, HPTN052 Antiretroviral Clinical Trial and the Metrogel intravaginal use Clinical Trial.
- Bonus Makanani MBBS MMed, Obstetrician and Gynecologist: Lecturer COM, University of Malawi: Co-investigator HPTN035 and Vaginal Microbicide Clinical Trial.
- Robin Broadhead MBBS FRCP, Pediatrician: Professor COM, University of Malawi: Co-Investigator HPTN024 and NVP/AZT Post-exposure Prophylaxis Clinical Trial.
- Frank Taulo MBBS, MPH, FCOG, Obstetrician and Gynecologist: Professor COM, University of Malawi: Co-investigator HPTN024 Perinatal Clinical Trial and NVP/AZT Post-Exposure Clinical Trial.
Literature Cited
- National Statistical Office, Zomba Malawi. Malawi Demographic and Health Survey 2000. Calverton, Maryland: ORC Macro. 2001.
- Ping L, Nelson JAE, Hoffman, IF, Schock J, Lamers AL, Goodman M, Vernazza P, Kazembe P, Maida M, Zimba D, Goodenow MM, Eron JJ, Fiscus SA, Cohen MS, Swanstrom R. Characterization of V3 sequence heterogeneity in Subtype C Human immunodeficiency Virus Type 1 isolates from Malawi: underepresentation of X4 variants. J Virol 1999; 73 (8): 6271-6281.
